Introduction: Reactivation of Epstein-Barr virus (EBV) is common in allogeneic hematopoietic cell transplantation (HCT). EBV infection leads to post-transplantation lymphoproliferative disease (PTLD), a life-threatening complication in this setting. Frequent molecular monitoring of viral load, especially in high risk patients and pre-emptive use of Rituximab has improved the outcome of EBV infection. However, the expansion of alternative transplants, leads to higher incidence and effective measures are warranted.
Methods: We have retrospectively studied the clinical characteristics of EBV reactivation in consecutive patients that underwent HCT between 2007-2019, when pre-emptive administration of Rituximab was a standard of care in our Unit and possible correlations were sought. EBV reactivation was considered when viral load >8500 viral genomic copies (VGC)/ml in whole blood was documented during regular molecular monitoring with RQ-PCR. Patients received treatment with Rituximab, at a scheme according to physician's decision. We considered undetectable levels as resolution of infection. Patients with PTLD proven by lymph node biopsy were treated as previously described by our group.
Results: Among 546 HCT recipients, EBV reactivation was detected in 100 patients, that suffered from hematologic malignancy (98) or aplastic anemia (2) and received grafts from matched sibling (23), unrelated (70) or haploidentical donors (12). Haploidentical donors were significantly higher in patients with EBV reactivation compared to our transplant population (12% versus 6%, p<0.001). Eighty-eight patients received myeloablative and 12/100 reduced intensity conditioning. Overall, EBV reactivation was detected at median 65 (20-2970) post-transplant days (median load: 26100, range 8690-2670000 VGC/ml). Rituximab was administered in 74 patients at median 4 (3-158) days post EBV reactivation. Most patients (63/74) received one cycle of Rituximab until undetectable EBV load. Rituximab cycles (median 1, range 0-3) were not associated with outcomes. Relapse of EBV reactivation was noted in 13/100 patients, with greater incidence among patients with later resolution of infection (27 vs 14 days in non relapsed, p<0.01). Late onset neutropenia related to Rituximab was noted in 16/74 patients and significantly correlated with increased EBV loads. Significantly higher viral loads were also noticed among patients who received ATG (44550 vs 20000 VGC/ml) or had haploidentical donors (60800 vs 22750 VGC/ml). Multivariate analysis confirmed that all above factors were independently associated to increased viral load. CMV concurrent reactivation was noted in 47 patients. Patients that received preemptive anti-CMV treatment presented with significantly delayed resolution of EBV infection, probably corresponding to greater immunosuppression.
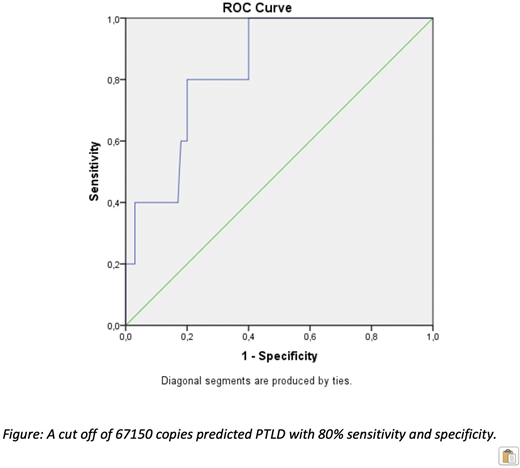
Five patients (two with haploidentical and three with unrelated donors) presented PTLD at 41 days post transplantation. ROC curve analysis identified a cut off of 67150 VGC/ml that predicts PLTD with 80% sensitivity and specificity (green line in Figure). Relapse free survival (RFS), overall survival (OS) and treatment-related mortality (TRM) in the entire cohort were similar regardless the EBV viral load or PTLD [4-year RFS 32.2%, 4-year OS 48.1% in a median follow up 29 months (4-216)]. ATG and chronic GVHD were independently associated with OS in the multivariate analysis (p<0.001, p<0.05 respectively). Similarly, ATG, chronic GVHD and age at transplant were independently associated to higher TRM in multivariate analysis (HR: 0.1, 1.16, 1.03, 95%CI: 0.15-0.5, 0.008-1.16, 1.007-1.05, respectively p<0.05). A trend for higher TRM was also noted among patients with EBV loads higher than 50000VGC/ml.
Conclusion: Our study indicates that regular monitoring and use of preemptive therapy is an effective strategy for prevention of EBV related complications. RFS and OS were not associated to severity of EBV reactivation. A useful cut off of EBV load for PTLD prevention was identified (67150 VGC/ml, specificity and sensitivity: 80%). However, expanding use of alternative transplants warrants a more effective treatment strategy. In this setting, use of specific antiviral cytotoxic cell lines could enhance viral specific cell mediated immunity and provide a better outcome in immunocompromised HCT recipients.
Gavriilaki:Omeros Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.